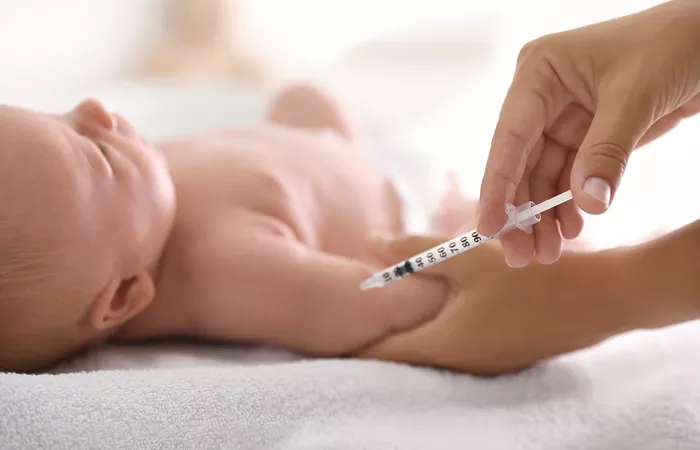
The U.S. Food and Drug Administration (FDA) approved Merck’s monoclonal antibody, Enflonsia, on Monday as a preventive treatment for infants up to one year old against respiratory syncytial virus (RSV) during their first RSV season.
Enflonsia, the first of its kind, is administered as a single dose, regardless of the infant’s birth weight. It is designed to protect healthy pre-term, full-term, and at-risk infants from mild, moderate, and severe cases of RSV. Merck confirmed that the treatment will be priced at $556 per dose.
RSV is a common virus that leads to seasonal illnesses like the flu. However, it is also a major cause of pneumonia and death among infants and elderly adults.
The approval of Enflonsia followed the results of a late-stage trial, which showed that its safety profile was similar to that of Synagis, a monthly injection developed by Swedish Orphan Biovitrum.
Addressing Dosing Challenges
Jefferies analyst Akash Tewari highlighted the advantages of Enflonsia’s single-dose regimen, especially compared to Beyfortus, the only other RSV preventive shot currently available for infants and toddlers in the U.S. Developed by Sanofi and AstraZeneca, Beyfortus requires dosing based on an infant’s weight during the RSV season, complicating the ordering and inventory processes. Merck’s Enflonsia, by contrast, simplifies this with a universal dose for all infants.
Supply and Production
The U.S. experienced limited availability of Beyfortus during the 2023 RSV season. However, Sanofi and AstraZeneca have since tripled production capacity and expanded their manufacturing sites. A Sanofi spokesperson confirmed that the current supply of Beyfortus matches the total amount distributed last season, with further increases expected as production continues.
The U.S. Centers for Disease Control and Prevention (CDC) reports that RSV leads to hospitalization for an estimated 58,000 to 80,000 children under five each year.
CDC Recommendations and Future Plans
The CDC currently offers two primary immunization options for infants to prevent severe RSV: an RSV vaccine administered to the mother during pregnancy, or an antibody shot given directly to the infant.
Merck anticipates that shipments of Enflonsia will be available in time for the 2025-2026 RSV season. The company also noted that the CDC’s Advisory Committee on Immunization Practices (ACIP) is scheduled to meet later this month to review and provide recommendations for the use of Enflonsia in infants.
However, Merck declined to comment on the recent dismissal of all members of the CDC panel by Health Secretary Robert F. Kennedy Jr.
Related topics: